-

Antioxidans Primum 1024
Proprietates Physicae Nomen Producti Antioxidans Primarium 1024 Nomen Chemicum duplex (3,5-ditert-butyl-4-hydroxy-phenylprenonyl)hydrazinum Nomen Anglicum Antioxidans Primarium Antioxidans 1024; bis(3,5-di-tert-Butyl-4-hydroxyhydrocinnamoyl)hydrazinum Numerus CAS 32687-78-8 Formula Molecularis C34H52N2O4 Pondus Molecularis 552.79 EINECS No. 251-156-3 Formula Structuralis Categoriae Conexae Catalysatores et Additiva; Antioxidans; Materiae Crudae Chemicae Organicae... -

HALS UV-770
Nomen producti: HALS UV-770
Nomen chemicum: Duplex (2,2,6,6-tetramethyl-4-piperidyl) decates
Nomen Anglicum: Stabilizator Lucis 770; Bis(2,2,6,6-tetramethyl-4-piperidyl)sebacas;
Numerus CAS: 52829-07-9
Formula molecularis: C28H52N2O4
Pondus moleculare: 480.72
Numerus EINECS: 258-207-9
Formula structuralis:
Categoriae conexae: Stabilisator lucis; absorbens ultraviolaceae; materiae rudis chemicae organicae; -
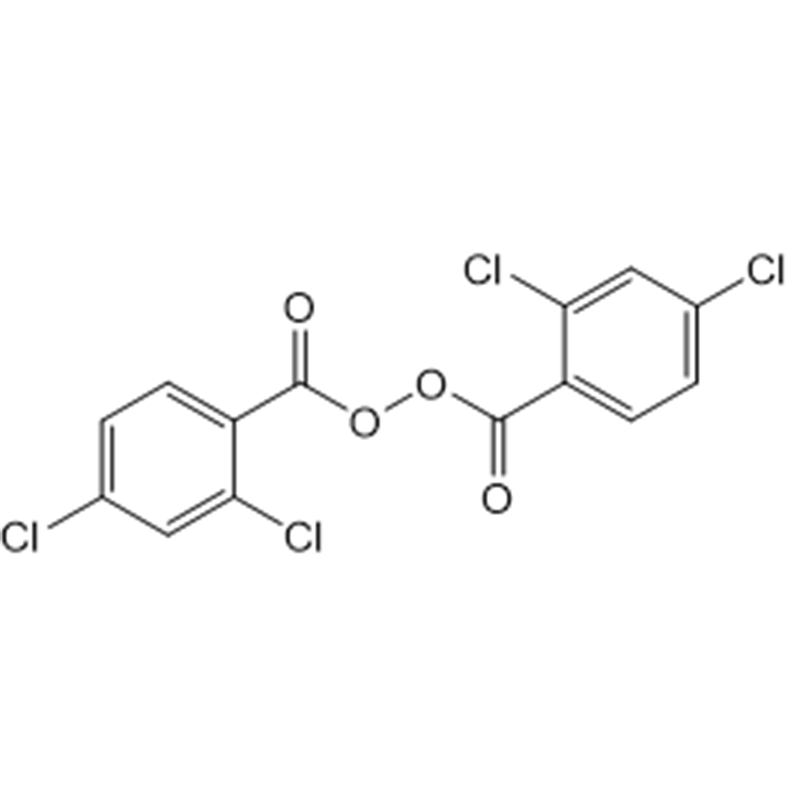
Peroxidum duplex (2,4-dichlorobenzolum) (pasta 50%)
Proprietates physicae Nomen producti 2,5-Dimethyl-2,5-di(tert-butylperoxy)hexanum Synonyma Trigonox 101; VAROX DBPH; VAROX DBPH-50; luperox; LUPEROX 101XL; Di-tert-butyl 1,1,4,4-tetramethyltetramethylene diperoxidum; 2,5-DIMETHYL-2,5-BIS(TERT-BUTYLPEROXY)HEXANE; 2,5-DIMETHYL-2,5-DI(T-BUTYL-PEROXY)HEXANE Numerus CAS 78-63-7 Formula molecularis C16H34O4 Pondus moleculare 290.44 Numerus EINECS 201-128-1 Formula structuralis Categoriae conexae oxidans, vulcan... -
![C41H39NO6 Acidum 1-pyrrolidincarboxylicum, 2-[[bis(4-methoxyphenyl)phenylmethoxy]methyl]-4-hydroxy-, 9H-fluoren-9-ylmethyl ester, (2S,4R)- (9Cl, ACI)](https://cdn.globalso.com/nvchem/C41H39NO6-1-Pyrrolidinecarboxylic-acid.jpg)
C41H39NO6 Acidum 1-pyrrolidincarboxylicum, 2-[[bis(4-methoxyphenyl)phenylmethoxy]methyl]-4-hydroxy-, 9H-fluoren-9-ylmethyl ester, (2S,4R)- (9Cl, ACI)
Proprietates Physicae Claves Proprietates Physicae Valor Conditio Pondus Moleculare 641.75 - Punctum Ebullitionis (Praedictum) 768.7±60.0 °C Pressio: 760 Torr Densitas (Praedicta) 1.237±0.06 g/cm3 Temperatura: 20 °C; Pressio: 760 Torr pKa (Praedictum) 14.50±0.40 Temperatura Acida Maxima: 25°C Alia Nomina et Identificatores SMILES Canonici O=C(OCC1C=2C=CC=CC2C=3C=CC=CC31)N4CC(O)CC4COC(C=5C=CC=CC5)(C6=CC=C(OC)C=C6)C7=CC=C(OC)C=C7 SMILES Isomerici C(OC[C@H]1N(C(OCC2C=3C(C=4C2=CC=CC4)=CC=CC3)=O)C[C@H](O)C1)(C5=CC=C(... -

C20H21NO4 Acidum 1-pyrrolidincarboxylicum, 4-hydroxy-2-(hydroxymethyl)-, 9H-fluoren-9-ylmethyl ester, (2S,4R)- (9Cl, ACI)
Proprietates Physicae Claves Proprietates Physicae Valor Conditio Pondus Moleculare 339.39 - Punctum Ebullitionis (Praedictum) 549.8±40.0 °C Pressio: 760 Torr Densitas (Praedicta) 1.318±0.06 g/cm3 Temperatura: 20 °C; Pressio: 760 Torr pKa (Praedictum) 14.53±0.40 Temperatura Acida Maxima: 25°C Alia Nomina et Identificatores SMILES Canonici O=C(OCC1C=2C=CC=CC2C=3C=CC=CC31)N4CC(O)CC4CO SMILES Isomerici C(OC(=O)N1[C@H](CO)C[C@@H](O)C1)C2C=3C(C=4C2=CC=CC4)=CC=CC3 InChI InChI=1S/C20H21NO4/c22-11-13-9-14(23)10-21(13)20... -
![C13H13NO5 1H-pyrano[3,4-f]indolizine-3,6,10(4H)-trione, 4-ethyl-7,8-dihydro-4- hydroxy-, (4S)- (9CI, ACI) H319, H302](https://cdn.globalso.com/nvchem/C13H13NO5-1H-Pyrano.jpg)
C13H13NO5 1H-pyrano[3,4-f]indolizine-3,6,10(4H)-trione, 4-ethyl-7,8-dihydro-4- hydroxy-, (4S)- (9CI, ACI) H319, H302
Proprietates Physicae Claves Proprietates Physicae Valor Conditio Pondus Moleculare 263.25 - Punctum Liquefactionis (Experimentale) 177.1-178.3 °C - Punctum Ebullitionis (Praedictum) 666.6±55.0 °C Pressio: 760 Torr Densitas (Praedicta) 1.50±0.1 g/cm3 Temperatura: 20 °C; Pressio: 760 Torr pKa (Praedictum) 11.20±0.20 Temperatura Acida Maxima: 25°C Alia Nomina et Identificatores SMILES Canonici O=C1C2=C(C=C3C(=O)CCN13)C(O)(C(=O)OC2)CC SMILES Isomerici C(C)[C@]1(O)C2=C(C(=O)N3C(=C2)C(=O)CC3)COC1=O InChI InChI=1S/C13H13NO5/c... -
![L-Ornithinamidum, L-valyl-N5-(aminocarbonyl)-N-[4-(hydroxymethyl)phenyl]-(9Cl, ACI) H335, H319, H315, H302](https://cdn.globalso.com/nvchem/L-Ornithinamide.jpg)
L-Ornithinamidum, L-valyl-N5-(aminocarbonyl)-N-[4-(hydroxymethyl)phenyl]-(9Cl, ACI) H335, H319, H315, H302
Proprietates Physicae Claves Proprietates Physicae Valor Conditio Pondus Moleculare 379.45 - Punctum Ebullitionis (Praedictum) 715.0±60.0 °C Pressio: 760 Torr Densitas (Praedicta) 1.243±0.06 g/cm3 Temperatura: 20 °C; Pressio: 760 Torr pKa (Praedictum) 13.75±0.46 Temperatura Acida Maxima: 25 °C Alia Nomina et Identificatores SMILES Canonici O=C(N)NCCCC(NC(=O)C(N)C(C)C)C(=O)NC1=CC=C(C=C1)CO SMILES Isomerici [C@@H](NC([C@H](C(C)C)N)=O)(C(NC1=CC=C(CO)C=C1)=O)CCCNC(N)=O InChI InChI=1S/C18H29N5O4/c1-11(2)15(19)17(26)23... -
![C33H39N5O6 L-Ornithinamidum, N-[(9H-fluoren-9-ylmethoxy)carbonyl]-L-valyl-N5-(aminocarbonyl)-N-[4-(hydroxymethyl)phenyl]-(9Cl, ACI)](https://cdn.globalso.com/nvchem/C33H39N5O6-L-Ornithinamide.jpg)
C33H39N5O6 L-Ornithinamidum, N-[(9H-fluoren-9-ylmethoxy)carbonyl]-L-valyl-N5-(aminocarbonyl)-N-[4-(hydroxymethyl)phenyl]-(9Cl, ACI)
Proprietates Physicae Claves Proprietates Physicae Valor Conditio Pondus Moleculare 601.69 - Punctum Ebullitionis (Praedictum) 914.2±65.0 °C Pressio: 760 Torr Densitas (Praedicta) 1.276±0.06 g/cm3 Temperatura: 20 °C; Pressio: 760 Torr pKa (Praedictum) 10.63±0.46 Temperatura Acida Maxima: 25 °C Alia Nomina et Identificatores SMILES Canonici O=C(OCC1C=2C=CC=CC2C=3C=CC=CC31)NC(C(=O)NC(C(=O)NC4=CC=C(C=C4)CO)CCCNC(=O)N)C(C)C SMILES Isomerici C(OC(N[C@H](C(N[C@H](C(NC1=CC=C(CO)C=C1)=O)CCCNC(N)=O)=O)[C@H](C)C)=O)C2C=3C(... -
![C21H23N3O5 L-Ornithinum, N5-(aminocarbonyl)-N2-[(9H-fluoren-9-ylmethoxy)carbonyl]- (9Cl, ACI)](https://cdn.globalso.com/nvchem/C21H23N3O5-L-Ornithine.jpg)
C21H23N3O5 L-Ornithinum, N5-(aminocarbonyl)-N2-[(9H-fluoren-9-ylmethoxy)carbonyl]- (9Cl, ACI)
Proprietates Physicae Claves Proprietates Physicae Valor Conditio Pondus Moleculare 397.43 - Punctum Ebullitionis (Praedictum) 671.5±55.0 °C Pressio: 760 Torr Densitas (Praedicta) 1.316±0.06 g/cm3 Temperatura: 20 °C; Pressio: 760 Torr pKa (Praedictum) 3.84±0.21 Temperatura Acida Maxima: 25 °C Alia Nomina et Identificatores SMILES Canonici O=C(OCC1C=2C=CC=CC2C=3C=CC=CC31)NC(C(=O)O)CCCNC(=O)N SMILES Isomerici C(OC(N[C@@H](CCCNC(N)=O)C(O)=O)=O)C1C=2C(C=3C1=CC=CC3)=CC=CC2 InChI InChI=1S/C21H23N3O5/c22-20(27)23-11-5-1... -
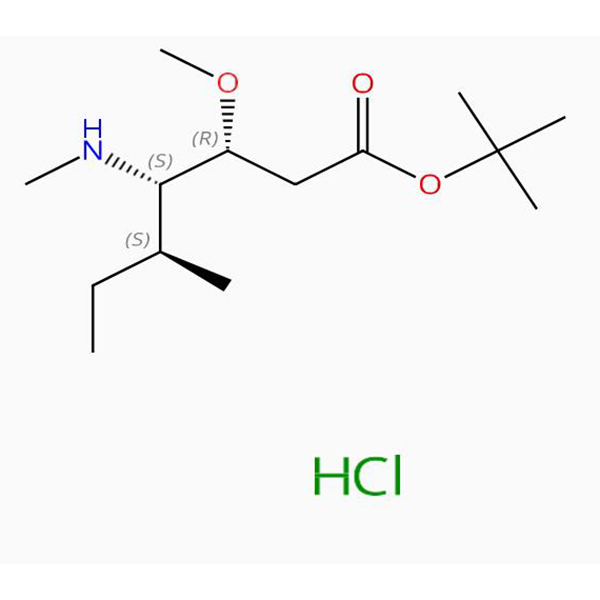
C14H29NO3.ClH Componentes: 2 Componentes RN: 474645-22-2 Acidum heptanoicum, 3-methoxy-5-methyl-4-(methylamino)-, 1,1-dimethylethyl ester, hydrochloridum (1:1), (3R,4S,5S)- (ACI)
Proprietates Physicae Clavis Proprietates Physicae Valor Conditio Pondus Moleculare 295.85 - Alia Nomina et Identificatores Canonica SMILES Cl.O=C(OC(C)(C)C)CC(OC)C(NC)C(C)CC Isomerica SMILES [C@@H]([C@@H](CC(OC(C)(C)C)=O)OC)([C@H](CC)C)NC.Cl InChI InChI=1S/C14H29NO3.ClH/c1-8-10(2)13(15-6)11(17-7)9-12(16)18-14(3,4)5;/h10-11,13,15H,8-9H2,1-7H3;1H/t10-,11+,13-;/m0./s1 Clavis InChI JRXGCIIOQALIMZ-LWEGJDAASA-N 2 Alia Nomina huius Substantiae Acidum heptanoicum, 3-methoxy-5-methyl-4-(methylamino)... -
![C20H31NO5 Acidum heptanoicum, 3-hydroxy-5-methyl-4-[[(phenylmethoxy)carbonyl]amino]-, 1,1-dimethylethyl ester, [3R-(3R*,4S*,5S*)]- (9Cl) H301](https://cdn.globalso.com/nvchem/C20H31NO5-Heptanoic-acid.jpg)
C20H31NO5 Acidum heptanoicum, 3-hydroxy-5-methyl-4-[[(phenylmethoxy)carbonyl]amino]-, 1,1-dimethylethyl ester, [3R-(3R*,4S*,5S*)]- (9Cl) H301
Proprietates Physicae Claves Proprietates Physicae Valor Conditio Pondus Moleculare 365.46 - Punctum Ebullitionis (Praedictum) 504.1±50.0 °C Pressio: 760 Torr Densitas (Praedicta) 1.091±0.06 g/cm3 Temperatura: 20 °C; Pressio: 760 Torr pKa (Praedictum) 11.82±0.46 Temperatura Acida Maxima: 25 °C Alia Nomina et Identificatores SMILES Canonici O=C(OCC=1C=CC=CC1)NC(C(O)CC(=O)OC(C)(C)C)C(C)CC SMILES Isomerici [C@H]([C@@H](CC(OC(C)(C)C)=O)O)(NC(OCC1=CC=CC=C1)=O)[C@H](CC)C InChI InChI=1S/C20H31NO5/c1-6-14(2)18(16(22)12-1... -
![118 Re36H44N2O8Si Uridinum, 5'-O-[bis(4-methoxyphenyl)phenylmethyl]-2'-O-[(1,1-dimethylethyl)dimethylsilyl]- (9CI, ACI)](https://cdn.globalso.com/nvchem/118-Re36H44N2O8Si-Uridine.jpg)
118 Re36H44N2O8Si Uridinum, 5'-O-[bis(4-methoxyphenyl)phenylmethyl]-2'-O-[(1,1-dimethylethyl)dimethylsilyl]- (9CI, ACI)
Proprietates Physicae Claves Proprietates Physicae Valor Conditio Pondus Moleculare 660.83 - Densitas (Praedicta) 1.24±0.1 g/cm3 Temperatura: 20 °C; Pressio: 760 Torr pKa (Praedictum) 9.39±0.10 Temperatura Acida Maxima: 25°C Alia Nomina et Identificatores SMILES Canonici O=C1C=CN(C(=O)N1)C2OC(COC(C=3C=CC=CC3)(C4=CC=C(OC)C=C4)C5=CC=C(OC)C=C5)C(O)C2O[Si](C)(C)C(C)(C)C SMILES Isomerici C(OC[C@H]1O[C@H]([C@H](O[Si](C(C)(C)C)(C)C)[C@@H]1O)N2C(=O)NC(=O)C=C2)(C3=CC=C(OC)C=C3)(C4=CC=C(OC)C=C4)C5=CC=CC= C5...

