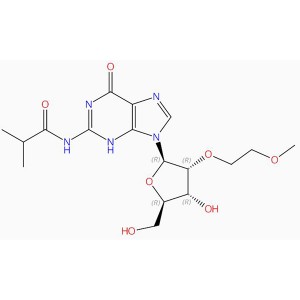
C47H60N7O10P Guanosinum, 5′-O-[bis(4-methoxyphenyl)phenylmethyl]-2′-O-(2-methoxyethyl)-N-(2-methyl-1-oxopropyl)-, 3′-[2-cyanoethyl N,N-bis(1-methylethyl)phosphoramiditum] (ACI)
Numerus Registri CAS
251647-55-9
H302
| Proprietates Physicae Claves | Valor | Conditio |
| Pondus Moleculare | 914.00 | - |
| pKa (Praedictum) | 9.16±0.20 | Temperatura Acida Maxima: 25°C |
Subrisus Canonici
N#CCCOP(OC1C(OC(N2C= NC=3C(=O)N=C(NC(=O)C(C)C)NC32)C1OCCOC)COC(C=4C=CC=CC4)(C5=CC=C(OC)C=C5)C6=CC=C(OC)C=C6) N(C(C)C)C(C)C
Subrisus Isomerici
C(OC[C@@H]¹[C@@H](OP(N(C(C)C)C(C)C)OCCC#N)[C@@H](OCCOC)[C@@H](O¹)N2C3=C(N=C2)C(=O)N=C(NC(C(C)C)=O)N3)(C4=CC=C (OC)C=C4)(C5=CC=C(OC)C=C5)C6=CC=CC=C6
InChI
InChI= 1S/C47H60N7O10P/c1-30(2)43(55)51-46-50-42-39(44(56)52-46)49-29-53(42)45-41(60-27-26-57-7)40(64-65(62-25-13-24-48)54( 31(3)4)32(5)6)38(63-45)28-61-47(33-14-11-10-12-15-33,34-16-20-36(58-8)21-17-34)35-18-22-37(59-9)23-19-35/h10-12,14-23,29-32,38,40-41,45H,13,25-28H2,1-9H3,(H2,50,51,52,55,56)/t38-,40-,41-,45-,65?/m1/s1
Clavis InChI
LADCDGNEBIQAAU-SBCRAQIVSA-N
XVII Alia Nomina Huius Substantiae
Guanosinum, 5′ -O- [bis(4-methoxyphenyl)phenylmethyl]-2′ -O-(2-methoxyethylum)-N-(2-methyl-1-oxopropyl)-, 3′-[2-cyanoethyl bis(1-methylethyl)phosphoramiditum] (9CI); 17: PN: US20030212017 PAGINA: 20 sequentia vindicata; 18: PN: US20030211606 PAGINA: 20
sequentia vindicata; 19: PN: US20040005569 PAGINA: 22 sequentia vindicata; 21: PN: US20040006030 PAGINA: 23 sequentia vindicata; 21: PN: US20040014047 PAGINA: 21 sequentia vindicata; 21: PN: US20040014049 PAGINA: 21 sequentia vindicata; 22: PN: US20030198965 PAGINA
E: 20 sequentia vindicata; 22: PN: US20040005570 PAGINA: 21 sequentia vindicata; 22: PN: US20040014048 PAGINA: 21 sequentia vindicata;
22: PN: US20040014050 PAGINA: 21 sequentia vindicata; 23: PN: US20040005565 PAGINA: 17-22 sequentia vindicata; 23: PN: US20040
014051 PAGINA: 23 sequentia vindicata; 24: PN: US20040014699 PAGINA: 21 sequentia vindicata; 25: PN: US20040006029 PAGINA: 23 sequentia vindicata; 25: PN: WO03106645 PAGINA: 73 sequentia vindicata; 96: PN: US20040005707 PAGINA: 21 sequentia vindicata
Spectra praesto sunt
13C NMR
Hetero NMR
Proprietates praesto
Biologicus
Chemica
Lipinski
Structurae Relata
| Possessio | Valor | Conditio | Fons |
| Factor Bioconcentrationis | 121 | pH 1; Temperatura: 25°C | (1) ACD |
| Factor Bioconcentrationis | 2580 | pH 2; Temperatura: 25°C | (1) ACD |
| Factor Bioconcentrationis | 23500 | pH 3; Temperatura: 25°C | (1) ACD |
| Factor Bioconcentrationis | 71800 | pH 4; Temperatura: 25°C | (1) ACD |
| Factor Bioconcentrationis | 89800 | pH 5; Temperatura: 25°C | (1) ACD |
| Factor Bioconcentrationis | 92100 | pH 6; Temperatura: 25°C | (1) ACD |
| Factor Bioconcentrationis | 91300 | pH 7; Temperatura: 25°C | (1) ACD |
| Factor Bioconcentrationis | 82500 | pH 8; Temperatura: 25°C | (1) ACD |
| Factor Bioconcentrationis | 42200 | pH 9; Temperatura: 25°C | (1) ACD |
| Factor Bioconcentrationis | 7860 | pH 10; Temperatura: 25°C | (1) ACD |
(1) Computatum utens Programmate Progressionis Chemiae Provectioris (ACD/Labs) V11.02 (© 1994-2023 ACD/Labs)
| Possessio | Valor | Conditio | Fons |
| Koc | CLXIV | pH 1; Temperatura: 25°C | (1) ACD |
| Koc | 3480 | pH 2; Temperatura: 25°C | (1) ACD |
| Koc | 31700 | pH 3; Temperatura: 25°C | (1) ACD |
| Koc | 96900 | pH 4; Temperatura: 25°C | (1) ACD |
| Koc | 1.21 × 105 | pH 5; Temperatura: 25°C | (1) ACD |
| Possessio | Valor | Conditio | Fons |
| Koc | 1.24 × 105 | pH 6; Temperatura: 25°C | (1) ACD |
| Koc | 1.23 × 105 | pH 7; Temperatura: 25°C | (1) ACD |
| Koc | 1.11 × 10⁵ | pH 8; Temperatura: 25°C | (1) ACD |
| Koc | 56900 | pH 9; Temperatura: 25°C | (1) ACD |
| Koc | 10600 | pH 10; Temperatura: 25°C | (1) ACD |
| logD | 3.95 | pH 1; Temperatura: 25°C | (1) ACD |
| logD | 5.28 | pH 2; Temperatura: 25°C | (1) ACD |
| logD | 6.24 | pH 3; Temperatura: 25°C | (1) ACD |
| logD | 6.73 | pH 4; Temperatura: 25°C | (1) ACD |
| logD | 6.82 | pH 5; Temperatura: 25°C | (1) ACD |
| logD | 6.83 | pH 6; Temperatura: 25°C | (1) ACD |
| logD | 6.83 | pH 7; Temperatura: 25°C | (1) ACD |
| logD | 6.79 | pH 8; Temperatura: 25°C | (1) ACD |
| logD | 6.50 | pH 9; Temperatura: 25°C | (1) ACD |
| logD | 5.77 | pH 10; Temperatura: 25°C | (1) ACD |
| logP | 6.837±0.764 | Temperatura: 25°C | (1) ACD |
| Solubilitas Intrinseca Massae | 4.4 × 10⁻⁴ g/L | Temperatura: 25°C | (1) ACD |
| Solubilitas Massae | 0.34 g/L | pH 1; Temperatura: 25°C | (1) ACD |
| Solubilitas Massae | 0.016 g/L | pH 2; Temperatura: 25°C | (1) ACD |
| Solubilitas Massae | 1.7 × 10⁻³ g/L | pH 3; Temperatura: 25°C | (1) ACD |
| Solubilitas Massae | 5.7 × 10⁻⁴ g/L | pH 4; Temperatura: 25°C | (1) ACD |
| Solubilitas Massae | 4.6 × 10⁻⁴ g/L | pH 5; Temperatura: 25°C | (1) ACD |
| Solubilitas Massae | 4.4 × 10⁻⁴ g/L | pH 6; Temperatura: 25°C | (1) ACD |
| Solubilitas Massae | 4.5 × 10⁻⁴ g/L | pH 7; Temperatura: 25°C | (1) ACD |
| Solubilitas Massae | 4.9 × 10⁻⁴ g/L | pH 8; Temperatura: 25°C | (1) ACD |
| Solubilitas Massae | 1.0 × 10⁻³ g/L | pH 9; Temperatura: 25°C | (1) ACD |
| Solubilitas Massae | 5.2 × 10⁻³ g/L | pH 10; Temperatura: 25°C | (1) ACD |
| Solubilitas Massae | 4.5 × 10⁻⁴ g/L | Aqua non tamponata pH 6.99; Temperatura: 25°C | (1) ACD |
| Solubilitas Intrinseca Molaris | 4.8 × 10⁻⁷ mol/L | Temperatura: 25°C | (1) ACD |
| Solubilitas Molaris | 3.7 × 10⁻⁴ mol/L | pH 1; Temperatura: 25°C | (1) ACD |
| Solubilitas Molaris | 1.7 × 10⁻⁵ mol/L | pH 2; Temperatura: 25°C | (1) ACD |
| Solubilitas Molaris | 1.9 × 10⁻⁶ mol/L | pH 3; Temperatura: 25°C | (1) ACD |
| Possessio | Valor | Conditio | Fons |
| Solubilitas Molaris | 6.2 × 10⁻⁷ mol/L | pH 4; Temperatura: 25°C | (1) ACD |
| Solubilitas Molaris | 5.0 × 10⁻⁷ mol/L | pH 5; Temperatura: 25°C | (1) ACD |
| Solubilitas Molaris | 4.8 × 10⁻⁷ mol/L | pH 6; Temperatura: 25°C | (1) ACD |
| Solubilitas Molaris | 4.9 × 10⁻⁷ mol/L | pH 7; Temperatura: 25°C | (1) ACD |
| Solubilitas Molaris | 5.4 × 10⁻⁷ mol/L | pH 8; Temperatura: 25°C | (1) ACD |
| Solubilitas Molaris | 1.1 × 10⁻⁶ mol/L | pH 9; Temperatura: 25°C | (1) ACD |
| Solubilitas Molaris | 5.7 × 10⁻⁶ mol/L | pH 10; Temperatura: 25°C | (1) ACD |
| Solubilitas Molaris | 4.9 × 10⁻⁷ mol/L | Aqua non tamponata pH 6.99; Temperatura: 25°C | (1) ACD |
| Pondus Moleculare | 914.00 | ||
| pKa | 9.16±0.20 | Temperatura Acida Maxima: 25°C | (1) ACD |
| pKa | 3.45±0.70 | Temperatura Maxima Fundamentalis: 25°C | (1) ACD |
(1) Computatum utens Programmate Progressionis Chemiae Provectioris (ACD/Labs) V11.02 (© 1994-2023 ACD/Labs)
| Possessio | Valor | Conditio | Fons |
| Obligationes libere rotabiles | 22 | (1) ACD | |
| Acceptores H | 17 | (1) ACD | |
| Donatores H | 2 | (1) ACD | |
| Summa Donatoris/Acceptoris H | 19 | (1) ACD | |
| logP | 6.837±0.764 | Temperatura: 25°C | (1) ACD |
| Pondus Moleculare | 914.00 |
(1) Computatum utens Programmate Progressionis Chemiae Provectioris (ACD/Labs) V11.02 (© 1994-2023 ACD/Labs)
| Possessio | Valor | Fons Conditionis |
| Area Superficialis Polaris | 203 A2 | (1) ACD |
(1) Computatum utens Programmate Progressionis Chemiae Provectioris (ACD/Labs) V11.02 (© 1994-2023 ACD/Labs)
Spectra praesto sunt
1H NMR
13C NMR
| Fons Enuntiationis Periculi Codicis | |
| H302 Nocivum si deglutitur. | Classificatio et Inscriptiones Agentiae Chemicae Europaeae (ECHA) Inventarium - Classificatio et inscriptiones notificatae - notificationes frequentissimae, Agentia Chemica Europaea (ECHA) Inventarium Classificationis et Inscriptionis - Classificatio et Inscriptionis Notificatae - Notificationes Gravissimae |

![C47H60N7O10P Guanosinum, 5′-O-[bis(4-methoxyphenyl)phenylmethyl]-2′-O-(2-methoxyethyl)-N-(2-methyl-1-oxopropyl)-, 3′-[2-cyanoethyl N,N-bis(1-methylethyl)phosphoramiditum] (ACI)](http://cdn.globalso.com/nvchem/style/global/img/demo/page_banner.jpg)
![C47H60N7O10P Guanosinum, 5′-O-[bis(4-methoxyphenyl)phenylmethyl]-2′-O-(2-methoxyethyl)-N-(2-methyl-1-oxopropyl)-, 3′-[2-cyanoethyl N,N-bis(1-methylethyl)phosphoramiditum] (ACI) Imago Praecipua.](https://cdn.globalso.com/nvchem/C47H60N7O10P-Guanosine.png)
![C47H60N7O10P Guanosinum, 5′-O-[bis(4-methoxyphenyl)phenylmethyl]-2′-O-(2-methoxyethyl)-N-(2-methyl-1-oxopropyl)-, 3′-[2-cyanoethyl N,N-bis(1-methylethyl)phosphoramiditum] (ACI)](https://cdn.globalso.com/nvchem/C47H60N7O10P-Guanosine-300x300.png)
![C41H41N5O8 Adenosinum, N-benzoyl-5'-O-[bis(4-methoxyphenyl)phenylmethyl]-2'-O-(2-methoxyethyl)- (9Cl, ACI)](https://cdn.globalso.com/nvchem/C41H41N5O8-Adenosine-300x300.jpg)
![C9H10N2O5 6H-Furo[2′,3′:4,5]oxazolo[3,2-a]pyrimidin-6-onum, 2,3,3a,9a-tetrahydro-3-hydroxy-2-(hydroxymethyl)-, (2R,3R,3aS,9aR)- (9Cl, ACI)](https://cdn.globalso.com/nvchem/C9H10N2O5-6H-Furo-300x300.png)

![C53H66N7O8PSi CAS NO.: 104992-55-4 Adenosinum, N-benzoyl-5′-O-[bis(4-methoxyphenyl)phenylmethyl]-2′-O-[(1,1-dimethylethyl)dimethylsilyl]-, 3′-[2-cyanoethyl N,N-bis(1-methylethyl)...](https://cdn.globalso.com/nvchem/C53H66N7O8PSi-300x300.png)